
If a galaxy’s spectrum shows many absorption lines and few emission lines, this suggests that its star-forming material has been depleted and that its stars are mainly old, while the opposite suggests it might be bursting with star formation and energetic stellar newborns. By hunting for specific signs of emission from various elements within a galaxy’s spectrum of light - so-called emission lines - or, conversely, the signs of absorption from other elements - so-called absorption lines - astronomers can start to deduce what might be happening within. This is done in much the same way as a glass prism spreads white light into its constituent wavelengths to create a rainbow.

One of these is to spread out the incoming light from that galaxy into a spectrum and explore its properties. When astronomers explore the contents and constituent parts of a galaxy somewhere in the Universe, they use various techniques and tools. The difference between emission and absorption spectra is that emission spectra always occur in a vacuum, while absorption spectra can occur in both vacuums and normal air.For this Picture of the Week, the NASA/ESA Hubble Space Telescope turned its powerful eye towards an emission line galaxy called NGC 3749. Absorption spectra are created when light passes through a material and the energy of the light is absorbed by the electrons in that material. In conclusion, emission spectra are produced when an electron loses energy while passing from a higher to a lower orbit. As a result, emission spectra contain all colors because all photons can be emitted, while absorption spectra only contain the colors that correspond to the energy levels of the atom’s electrons. These energies determine the colors that are absorbed. In this state, the atom can only absorb photons that have energies corresponding to the energy levels of its electrons. On the other hand, when an atom absorbs light, it is said to be in an excited state. The energy of the photon will determine its color. When this happens, the atom will release a photon, which is a particle of light. The reason for this difference is that, in order for an atom to emit light, it must first be excited, meaning that its electrons must be raised to a higher energy level. The difference between the two is that emission spectra contain all the colors of the visible spectrum, while absorption spectra have dark lines corresponding to the colors that are absorbed. Emission spectra are created when atoms emit light, while absorption spectra are created when atoms absorb light. The Absorption Spectra can also be used to study the interaction of the material with other materials.ĭifference between Emission and Absorption SpectraĮmission and absorption spectra are two types of atomic spectra. The Absorption Spectra can also be used to determine the physical and chemical properties of the material. The Absorption Spectra can also be used to quantitatively analyze the concentrations of these chemicals. The Absorption Spectra is used to identify the chemicals present in a sample. The material can be a gas, liquid, or solid. What is Absorption Spectra?Ībsorption spectra is the selective absorption of electromagnetic radiation of a particular wavelength by a material. In addition, emission spectra can be used to study chemical reactions and analyze the composition of substances.
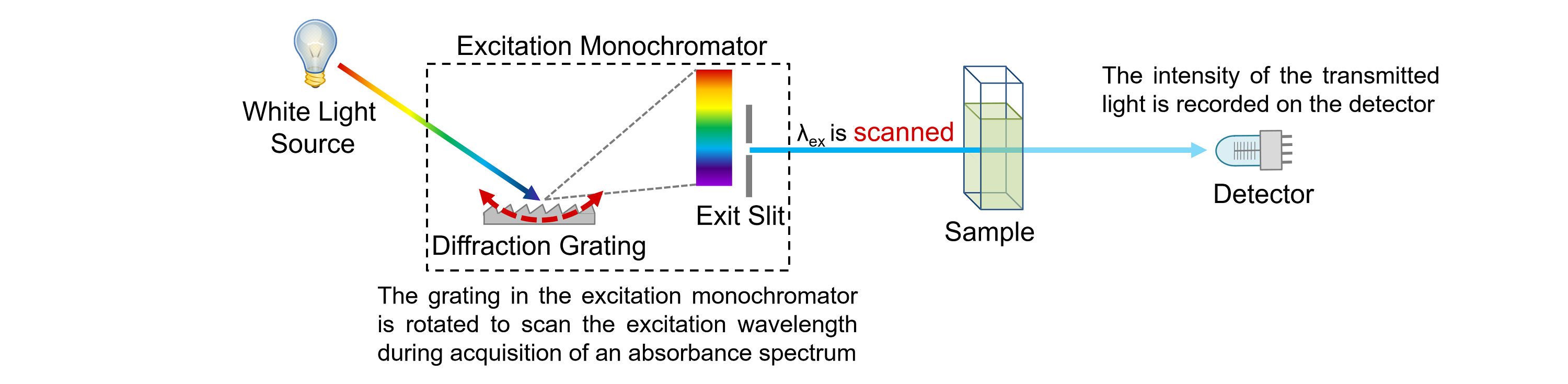
emission spectra can also be used to identify elements in stars and other objects in the universe. By studying the emission spectra of different elements, scientists can learn about the structure of atoms and molecules. Emission spectra are used in a variety of fields, such as astronomy and chemistry. This radiation makes up the emission spectrum. When the excited electrons return to their lower energy state, they emit radiation at specific wavelengths. The spectrum is produced when the electrons in the atom or molecule absorb energy, such as heat or light, and are excited to a higher energy state. The emission spectrum is unique to each element, and can be used to identify the element. Emission spectra are the radiation emitted by an atom or molecule when it undergoes a transition from an excited state to a lower energy state.


 0 kommentar(er)
0 kommentar(er)
